Published IH and IHC Pictures for 26905 Cdc42-GTP mAb - Part 012

Mammalian antibodies and immunological methods
Fixation and processing of cells and mouse tissue sections was as previously described (Zihni et al., 2017). The following antibodies were used: RPE65 mouse monoclonal (Millipore) 1/300 for immuno-fluorescence; p-MLC S19, mouse monoclonal (Cell Signaling Technology) immunofluorescence 1/100 and immunoblotting 1/1,000; Dbl(3), rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200; MRCKβ, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/500; anti–Cdc42-GTP mouse monoclonal (NewEast Biosciences) immunofluorescence 1/50; N-WASP, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/1,000; MerTK rabbit monoclonal (52968; Abcam; recognizes an extracellular N-terminal epitope) immunofluorescence 1/100; IntegrinαVβ5, mouse monoclonal (P1F6; Clone; 24694; Abcam) immunofluorescence 1/100; pFAK Y397, rabbit polyclonal (Invitrogen) immunofluorescence 1/100; pSrc Y416, rabbit polyclonal (Cell Signaling Technology) immunofluorescence 1/100; Phalloidin-Atto 647 reagent was obtained from Sigma-Aldrich and diluted 1/1,000. Affinity-purified and cross-adsorbed Alexa488-, Cy3- and Cy5-labeled donkey anti-mouse, rabbit, or goat secondary antibodies were from Jackson ImmunoResearch Laboratories (1/300 diluted from 50% glycerol stocks). Affinity-purified HRP-conjugated goat anti-mouse and rabbit, and donkey anti-goat secondary antibodies 1/5,000 were also from Jackson ImmunoResearch Laboratories (1/5,000 diluted from 50% glycerol stocks). For immunofluorescence analysis, cells and tissues were mounted using Prolong Gold antifade reagent (Life Technologies), and imaging was performed using Zeiss 700 and 710 confocal microscopes and a 64× oil lens/NA1.4. Images were processed using Zeiss Zen2009 and Adobe Photoshop CS5 and 10 software. Coimmunoprecipitation and immunoblotting were carried out using methods previously described and were repeated at least three times (Zihni et al., 2014; Zihni et al., 2017).
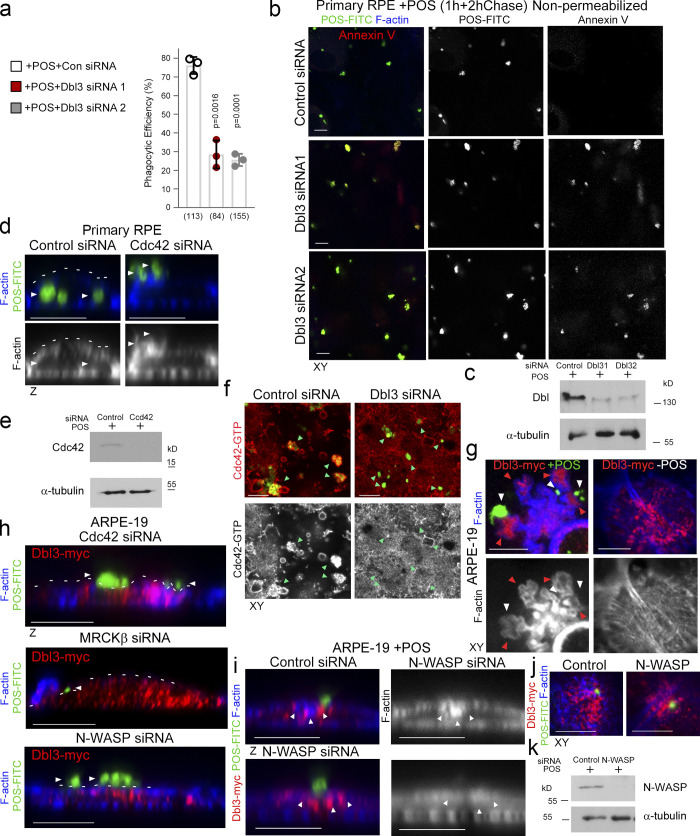
Apical Cdc42 signaling drives cup formation and engulfment in the RPE.
(a–e) …… (k–m) POS-membrane contact rapidly induces membrane protrusions enriched in the Cdc42 GEF Dbl3, active Cdc42-GTP, and the Cdc42 effectors MRCKβ and N-WASP. Quantifications: means ±1SD of n = 3 independent experiments; indicated are the total number of cells analyzed and P values derived from t tests.
Mammalian antibodies and immunological methods
Fixation and processing of cells and mouse tissue sections was as previously described (Zihni et al., 2017). The following antibodies were used: RPE65 mouse monoclonal (Millipore) 1/300 for immuno-fluorescence; p-MLC S19, mouse monoclonal (Cell Signaling Technology) immunofluorescence 1/100 and immunoblotting 1/1,000; Dbl(3), rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200; MRCKβ, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/500; anti–Cdc42-GTP mouse monoclonal (NewEast Biosciences) immunofluorescence 1/50; N-WASP, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/1,000; MerTK rabbit monoclonal (52968; Abcam; recognizes an extracellular N-terminal epitope) immunofluorescence 1/100; IntegrinαVβ5, mouse monoclonal (P1F6; Clone; 24694; Abcam) immunofluorescence 1/100; pFAK Y397, rabbit polyclonal (Invitrogen) immunofluorescence 1/100; pSrc Y416, rabbit polyclonal (Cell Signaling Technology) immunofluorescence 1/100; Phalloidin-Atto 647 reagent was obtained from Sigma-Aldrich and diluted 1/1,000. Affinity-purified and cross-adsorbed Alexa488-, Cy3- and Cy5-labeled donkey anti-mouse, rabbit, or goat secondary antibodies were from Jackson ImmunoResearch Laboratories (1/300 diluted from 50% glycerol stocks). Affinity-purified HRP-conjugated goat anti-mouse and rabbit, and donkey anti-goat secondary antibodies 1/5,000 were also from Jackson ImmunoResearch Laboratories (1/5,000 diluted from 50% glycerol stocks). For immunofluorescence analysis, cells and tissues were mounted using Prolong Gold antifade reagent (Life Technologies), and imaging was performed using Zeiss 700 and 710 confocal microscopes and a 64× oil lens/NA1.4. Images were processed using Zeiss Zen2009 and Adobe Photoshop CS5 and 10 software. Coimmunoprecipitation and immunoblotting were carried out using methods previously described and were repeated at least three times (Zihni et al., 2014; Zihni et al., 2017).
Cdc42 drives protrusion induction and cup maturation. (a–c)…. (f) Active Cdc42-GTP at nascent protrusions is inhibited by siRNA-mediated knockdown of Dbl3. Note, green arrowheads highlight POS-membrane bound sites.

Mammalian antibodies and immunological methods
Fixation and processing of cells and mouse tissue sections was as previously described (Zihni et al., 2017). The following antibodies were used: RPE65 mouse monoclonal (Millipore) 1/300 for immuno-fluorescence; p-MLC S19, mouse monoclonal (Cell Signaling Technology) immunofluorescence 1/100 and immunoblotting 1/1,000; Dbl(3), rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200; MRCKβ, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/500; anti–Cdc42-GTP mouse monoclonal (NewEast Biosciences) immunofluorescence 1/50; N-WASP, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/1,000; MerTK rabbit monoclonal (52968; Abcam; recognizes an extracellular N-terminal epitope) immunofluorescence 1/100; IntegrinαVβ5, mouse monoclonal (P1F6; Clone; 24694; Abcam) immunofluorescence 1/100; pFAK Y397, rabbit polyclonal (Invitrogen) immunofluorescence 1/100; pSrc Y416, rabbit polyclonal (Cell Signaling Technology) immunofluorescence 1/100; Phalloidin-Atto 647 reagent was obtained from Sigma-Aldrich and diluted 1/1,000. Affinity-purified and cross-adsorbed Alexa488-, Cy3- and Cy5-labeled donkey anti-mouse, rabbit, or goat secondary antibodies were from Jackson ImmunoResearch Laboratories (1/300 diluted from 50% glycerol stocks). Affinity-purified HRP-conjugated goat anti-mouse and rabbit, and donkey anti-goat secondary antibodies 1/5,000 were also from Jackson ImmunoResearch Laboratories (1/5,000 diluted from 50% glycerol stocks). For immunofluorescence analysis, cells and tissues were mounted using Prolong Gold antifade reagent (Life Technologies), and imaging was performed using Zeiss 700 and 710 confocal microscopes and a 64× oil lens/NA1.4. Images were processed using Zeiss Zen2009 and Adobe Photoshop CS5 and 10 software. Coimmunoprecipitation and immunoblotting were carried out using methods previously described and were repeated at least three times (Zihni et al., 2014; Zihni et al., 2017).
POS-membrane contacts induce membrane deformation.
(a–d) Confocal z-section analysis reveals that POS binding to the apical membrane of RPE, induces membrane protrusions rich in Dbl3, Cdc42-GTP, MRCKβ, and N-WASP. White dashes highlight the normal apical membrane.
Mammalian antibodies and immunological methods
Fixation and processing of cells and mouse tissue sections was as previously described (Zihni et al., 2017). The following antibodies were used: RPE65 mouse monoclonal (Millipore) 1/300 for immuno-fluorescence; p-MLC S19, mouse monoclonal (Cell Signaling Technology) immunofluorescence 1/100 and immunoblotting 1/1,000; Dbl(3), rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200; MRCKβ, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/500; anti–Cdc42-GTP mouse monoclonal (NewEast Biosciences) immunofluorescence 1/50; N-WASP, rabbit polyclonal (Santa Cruz Biotechnology) immunofluorescence 1/200 and immunoblotting 1/1,000; MerTK rabbit monoclonal (52968; Abcam; recognizes an extracellular N-terminal epitope) immunofluorescence 1/100; IntegrinαVβ5, mouse monoclonal (P1F6; Clone; 24694; Abcam) immunofluorescence 1/100; pFAK Y397, rabbit polyclonal (Invitrogen) immunofluorescence 1/100; pSrc Y416, rabbit polyclonal (Cell Signaling Technology) immunofluorescence 1/100; Phalloidin-Atto 647 reagent was obtained from Sigma-Aldrich and diluted 1/1,000. Affinity-purified and cross-adsorbed Alexa488-, Cy3- and Cy5-labeled donkey anti-mouse, rabbit, or goat secondary antibodies were from Jackson ImmunoResearch Laboratories (1/300 diluted from 50% glycerol stocks). Affinity-purified HRP-conjugated goat anti-mouse and rabbit, and donkey anti-goat secondary antibodies 1/5,000 were also from Jackson ImmunoResearch Laboratories (1/5,000 diluted from 50% glycerol stocks). For immunofluorescence analysis, cells and tissues were mounted using Prolong Gold antifade reagent (Life Technologies), and imaging was performed using Zeiss 700 and 710 confocal microscopes and a 64× oil lens/NA1.4. Images were processed using Zeiss Zen2009 and Adobe Photoshop CS5 and 10 software. Coimmunoprecipitation and immunoblotting were carried out using methods previously described and were repeated at least three times (Zihni et al., 2014; Zihni et al., 2017).
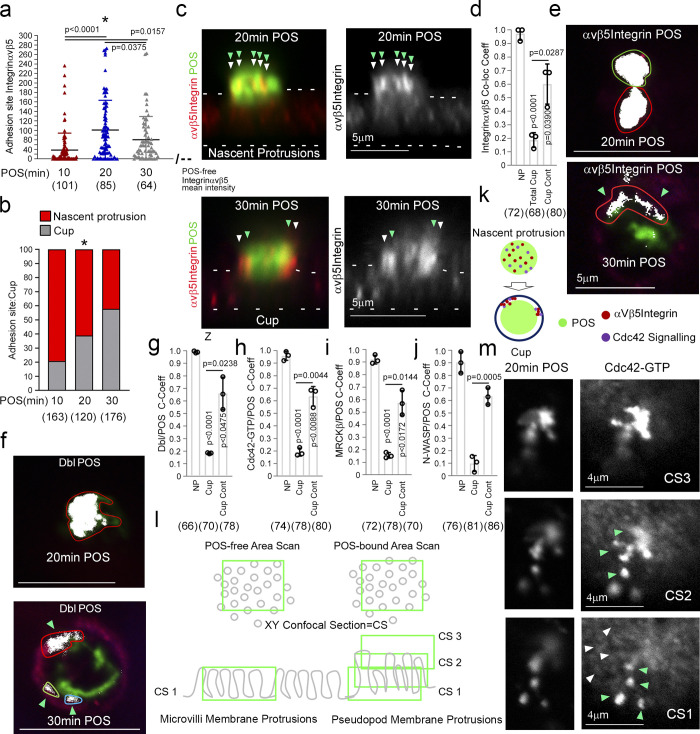
POS binding induces rapid recruitment of Cdc42 signaling components.
(a–j) Integrin αvβ5 and Cdc42 signaling components recruitment to POS adhesion sites upon 10 (early adhesion), 20 (membrane deformation and protrusions), and 30 min (cups). Note, POS binding to the integrin αvβ5 receptor induces rapid colocalization of Dbl3, Cdc42-GTP, MRCKβ, and N-WASP with the POS–integrin complex. Nascent protrusions (white arrowheads) display almost complete colocalization of Cdc42-signaling components with POS (highlighted by green arrowheads) whereas in the cup conformation (white arrowheads) colocalization occurs at discrete contact sites (green arrowheads) at the cup–POS interface. (k) Schematic diagram illustrating almost complete co-localization of integrinαvβ5 and Ccd42-signaling proteins with POS at nascent protrusions and partial co-localization at POS-cup adhesion sites. (l) Schematic diagram illustrating method of quantifying mean labeling intensity of protrusion proteins using Cdc42-GTP as an example. Confocal xy sections were scanned at the apical membrane surface of RPE cells (Confocal Scan 1, CS1), both unattached and POS attached. Since protrusions are at an increased height compared to normal apical brush border microvilli, further serial scans were taken as appropriate to cover the protrusion length and values were averaged. (m) At CS1, Cdc42-GTP enrichment is observed at single protrusions (green arrowheads) at a similar diameter to surrounding non-POS attached brush border structures (white arrowheads). Quantifications: means ±1SD of n = 3 independent experiments; indicated are the total number of cells analyzed and P values derived from t tests, except for the scatter plot in panel a where the median and upper and lower quartiles are highlighted, and n values represent number of cells collected from three independent experiments, and significance was tested with an ANOVA test.
Immunochemical detection of proteins in brain slices and cultured cells
Cellular immunostaining was carried out as described in (He et al. 2005). Briefly, cultured neurons were fixed in 1× PEM (80 mM PIPES, 5 mM EGTA, and 1 mM MgCl2, pH 6.8) containing 0.3 % glutaraldehyde and then permeabilized at room temperature in 0.05% Triton-X 100 prior to glutaraldehyde quenching with 10 mg/ml sodium borohydride. Cells were blocked in 10 mg/ml BSA + 10% goat serum prior to being immunostained overnight at 4°C with the following primary Abs: mouse anti-Tau-1; anti-Gprin1; pGAP-43; GTP-CDC42 (New East). Secondary Abs used (all purchased from Jackson-ImmunoResearch): carbocyanine (Cy) 2/3; Dylight 488; Dylight 560; and AlexaFluor 647. α7 were visualized using fBgtx.
Western blot detection was obtained using nitrocellulose membranes blocked with 5% nonfat milk (or 1% BSA for biotinylation experiments). Membranes were probed with the following primary Abs overnight at 4°C: α7 nAChR (SC); Gprin1; HCN1 (SC); GAP-43 (Abcam); pGAP-43; CDC42 (SC); GTP-CDC42; GAPDH (Cell Signaling). Species-specific peroxidase conjugated secondary Abs were purchased from (Jackson- Immunoresearch). Signals were detected using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) with SeeBlue and MagicMark (Invitrogen) as molecular weight standards. Blots were imaged using the Gel Doc Imaging system (Bio-Rad). Band density analysis was performed using ImageJ version 10.2 (NIH). Western blot values are based on averages from three separate independent experiments.
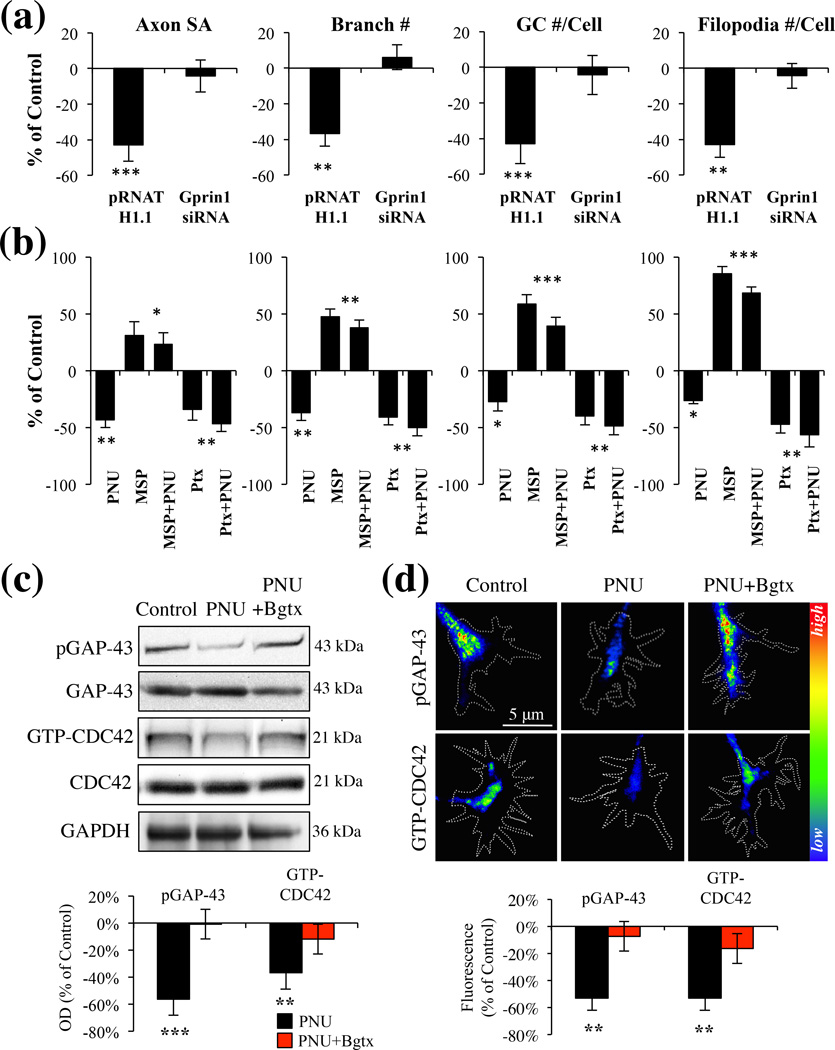
α7 mediated inhibition of axon growth is dependent on a Gprin1 pathway. (a) An analysis of axon and GC morphology in cells treated with 10 µM PNU. Values are based on average percent change in axon growth from control cells (cells transfected with the same plasmid but treated with 0.1% DMSO). (b) An analysis of axon and GC morphology in cells treated with: PNU (10 µM); Ptx (1 µM), mastoparan (30 µM) Values are based on average change in axon growth from control cells which were treated with 0.1% DMSO alone. (c–d) Analysis of pGAP-43 and GTP-CDC42 expression within hippocampal neurons treated with PNU (10 µM), PNU+Bgtx (50 nM), or Control (0.1% DMSO) for 60 min in MP (c) and GCs (d). Immunofluoresecence signal in d represented as a heat map.
Calcium switch assay and immunofluorescence staining
HUVECs serum starved for 10 h in EBM2 (Lonza) starvation medium containing 0.2% fetal bovine serum were challenged with 4 mM ethylene glycol tetraacetic acid (EGTA) for 30 min to chelate extracellular calcium and disrupt Ca2+-dependent intercellular junctions. After washing, cells were allowed to recover in calcium-containing EBM2 complete culture medium for various intervals. Cells were fixed and permeabilized with 3.7% paraformaldehyde and 0.25% Triton X-100 in cytoskeletal buffer (10 mM 2-(N-morpholino)ethanesulfonic acid, 150 mM NaCl, 5 mM EGTA, 5 mM MgCl2, and 5 mM glucose (pH 6.1) for 10 min at room temperature and subsequently processed for immunofluorescence.
Immune detection of endogenous proteins was performed as previously described (Rivera et al., 2004). After fixation and permeabilization, endothelial monolayers were blocked with 2% bovine serum albumin (BSA) in PBS (1 h) before overnight incubation with primary antibody (rabbit polyclonal anti–VE-cadherin antibody, 1:1000 [2012-02; Bender Med System], and anti–human podocalyxin antibody, 1:500 [AF 1658; R&D Systems]). Cells were then extensively washed to remove the unbound excess primary antibody and subsequently incubated with goat anti-rabbit immunoglobulin G (IgG)–horseradish peroxidase (HRP; 1:1000; sc-2054; Santa Cruz Biotechnology) containing DAPI (5 ng/ml) and Texas red phalloidin (1:100; Invitrogen) for 1
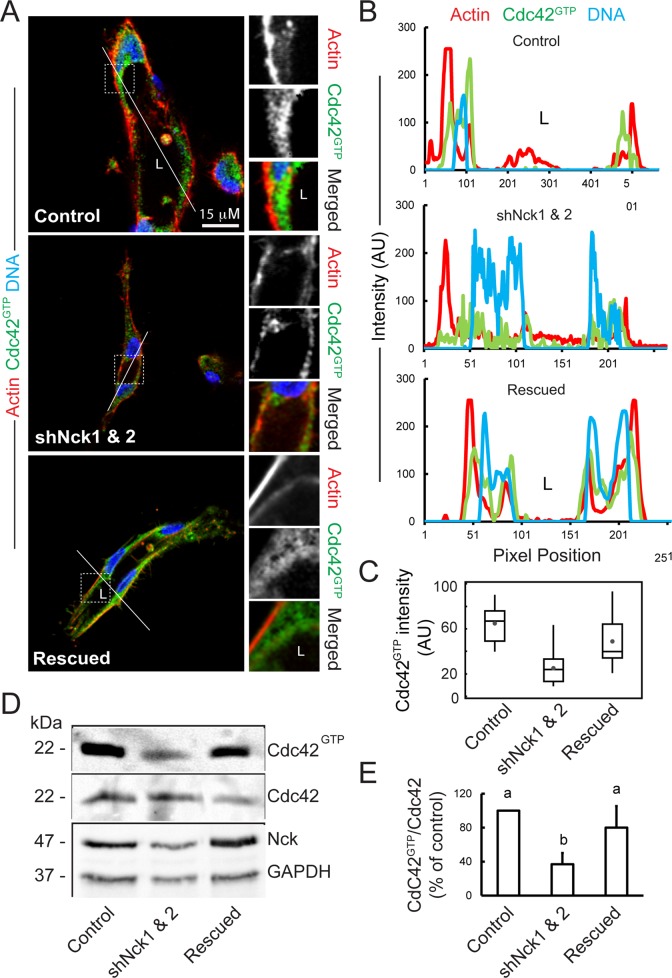
Loss of Nck disrupts activation of endogenous Cdc42 in cells cultured in 3D collagen matrices.
(A) Confocal images showing the cytoskeletal architecture (F-actin; red), distribution of GTP-bound Cdc42 (Cdc42GTP; green), and nuclei (DNA; blue). Lumens (L) are indicated. The ROIs (dotted squares) were magnified and are shown to the right. Scale bar, 15 μm. (B) Line scans showing intensities along the solid white lines displayed in A. (C) Quantification of luminal Cdc42 fluorescence intensity. Average pixel intensity was extracted from 35–50 ROIs from images acquired in three independent experiments. (D) Representative Western blots showing levels of GTP-bound Cdc42 (PBDPak1 pull-down assays), total Cdc42, Nck, and GAPDH in cell extracts obtained from 3D cultures. (E) Quantification of active Cdc42. The intensity of bands corresponding to active Cdc42 (PBDPak1 pull-down assays) was normalized by the intensity of bands corresponding to total Cdc42 in cell extracts and expressed as percentage of control cultures (n = 3 independent experiments).



