Published Pictures for 26913 Rap1-GTP mAb
Immunoprecipitation and RalGTPase activity
RalGTPase activity in vivo was assessed using the Ral activation assay (New East Bioscience) following product guidance with some modifications. Homogenates of a pool of two sciatic nerves from two different animals, to generate each data point, with immunoprecipitation Lysis Buffer (50 mM Tris-HCl, 125 mM NaCl, 1% NP-40, 0.1% SDS, and anti-proteases), were incubated for 1 h at RT with magnetic beads (Bio-Rad) previously incubated with 1 µg of anti-active Ral antibody (New East Bioscience) for 10 min. To elute the antigen from the bead-antibody complex, the beads were incubated 10 min with 40 µl of 1× Laemmli buffer at 70°C. Immunoprecipitation products were then loaded into 12% Mini-PROTEAN TGX Precast Protein Gels (Bio-Rad) and transferred to an Immobilon-P Membrane, polyvinylidene fluoride, 0.45-µm membranes (Millipore). Finally, membranes were incubated with antibodies against anti-RalA or -RalB, as described in the Western blotting section, to detect activated forms of RalA and RalB, respectively.
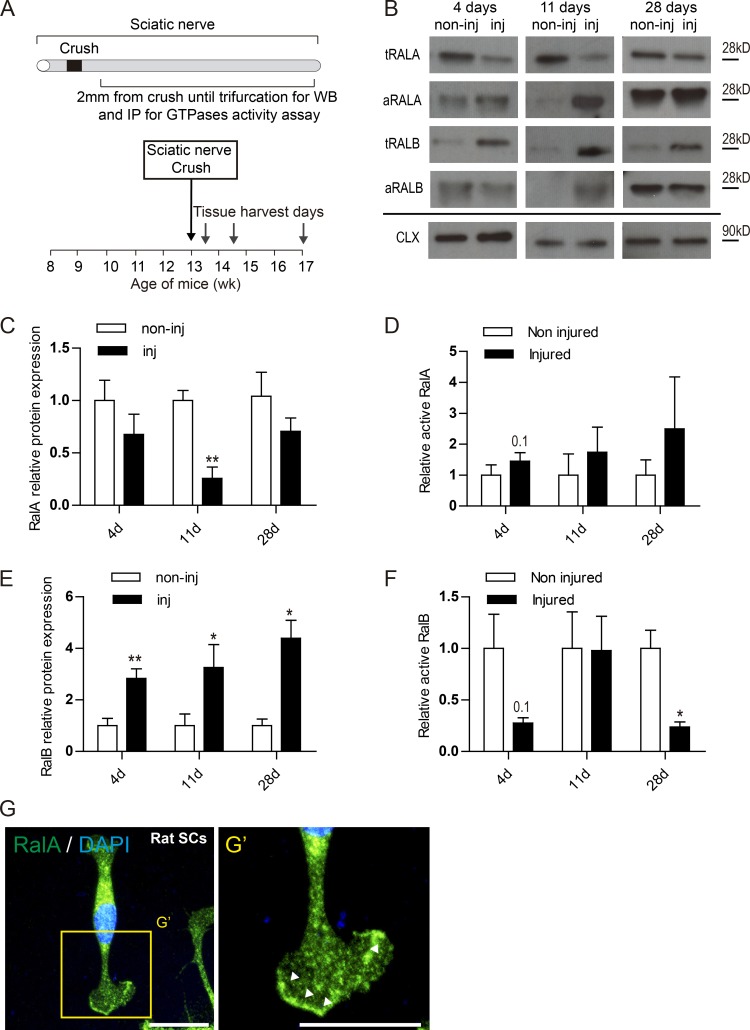
Dysregulation of RalGTPase expression, activation after nerve injury and their expression in SCs in vitro.
(A) Schematic representation of the sciatic nerve crush model used to assess the role of RalGTPases on nerve repair. (B) Western blot of injured (inj) and noninjured (non-inj) sciatic nerves 4, 11, and 28 d after sciatic nerve crush in WT mice probed with antibodies against RalA and RalB. Calnexin (CAL) used as loading control. Immunoprecipitation blot of activated forms of RalA and RalB. (C–F) Quantification of the relative protein expression of RalA (C) and RalB (E) across the time points analyzed in the Western blots. Quantification of the relative protein expression of active RalA (D) and active RalB (F) across the time points. Data are presented as fold change against noninjured relative expression (**, P < 0.01; *, P < 0.05; Student’s t test), n = 4 or 5. Data are presented as mean ± SEM. (G) Primary cultures of rat SCs demonstrate expression of endogenous RalA (green) in the nucleus, perinuclear cytoplasm, and in the plasma membrane. (G’) High magnification picture shows an enrichment of RalA in the membrane edge of lamellipodia (arrow bars). Scale bars, 50 µm.
Immunoprecipitation
For large-scale endogenous protein immunoprecipitations, five 150 cm dishes per experimental condition were grown to confluence. Cells were washed on ice with PBS and collected in non-denaturing lysis buffer (20 mM Tris-HCl pH 7.4, 137mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 10mM MgCl2, 2mM EGTA, Roche EDTA-free complete ULTRA protease inhibitor and Roche PhosphoSTOP). Cells were incubated with mild agitation for 1 hour at 4°C and cleared at 16,000 X g for 10 min at 4°C. 2mg of lysate was brought to a concentration of 1.5mg/mL by dilution with fresh lysis buffer. Immunoprecipitations were carried using 2.5mg of antibody per sample. For endogenous and single tagged proteins this was followed by 2–18 hour precipitation of antibody-antigen complexes (as optimized for each antibody) using 90μl of Protein A/G-agarose beads (Santa Cruz Biotechnology, SC-2003). Subsequently, complexes were washed in lysis buffer 4 times at 4°C and eluted with 90μl 2X SDS sample buffer (BIORAD Catalog#161–0737) at 95°C for 12 min.
For overexpression IP, 3X106 HEK293T cells were seeded, in four 10cm dishes per condition, in DMEM + 10% FBS. The following day, cells were transfected with 3μg plasmid using Fugene 6 at a ratio of 3:1 (mL Fugene 6 to mg DNA). 48 hours post transfection, cells were collected in non-denaturing lysis buffer (20 mM Tris-HCl pH 8.0, 100mM KCl, 0.1% NP-40, 0.5% sodium deoxycholate, 10mM MgCl2, 2mM EDTA) with protease and phosphatase inhibitors (Roche EDTA-free cOmplete ULTRA and PhosphoSTOP) and lysates prepared as above. Endogenous IP, for when RALB is a target, had to be performed either with 0.4mM EDTA or in presence of ectopic overexpression of RALB.
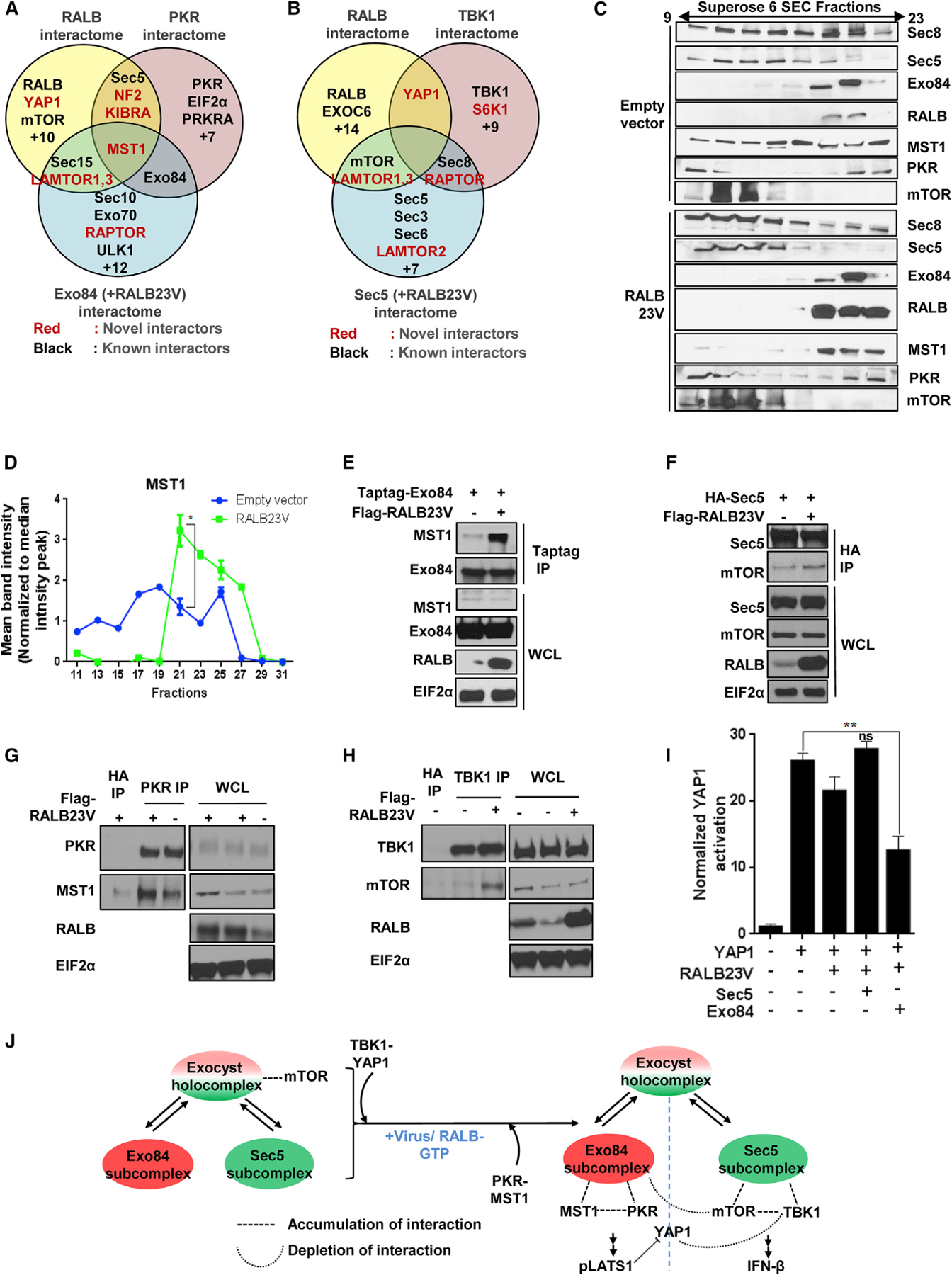
RALB drives formation of Exo84/PKR/MST1 and Sec5/TBK1/mTOR complexes
(E) Active RALB promotes Exo84-MST1 interaction. Exo84 was immunoprecipitated from HEK293T cells transiently expressing Myc-Exo84 with or without FLAG-RALB and analyzed using SDS-PAGE (n = 2).
(F) Active RALB promotes Sec5-mTOR interaction. Sec5 was immunoprecipitated from HEK293T cells transiently expressing HA-Sec5 with or without FLAG-RALB and analyzed using SDS-PAGE (n = 2).



